USP WFI is frequently made in a consistently circulating system maintained at an elevated temperature. The significant temperature, preserved uniformly all over the system by consistent circulation, stops major microbial progress. A temperature of eighty^oC is commonly used and is appropriate.
Right after installing the media materials and connecting the mandatory interfaces, expert experts can accomplish a quick startup. The water remedy system is then ready to use, is often rented for many days or perhaps weeks, and is not hard to remove. Even so, these remedies usually are not supposed for GMP-appropriate programs.
Updates: All regulatory modifications are lined, slight or massive. By using a substantial frequency update fee of 6x a 12 months, you will be always current.
PharmaState.academy presents easy accessibility to training & up-skilling packages designed by authorities from Pharma Business.
The topic of this post is especially the purified water generation unit. Storage and distribution systems need to be put in inside the production creating, and the water purification unit is connected to an existing tank. Attainable alternatives are revealed in the shots on.
6.two Crucial running parameters really should be discovered. Reports around the important variables ought to include things like ailments encompassing higher and decrease operating limits and conditions (also known as “worst situation ailments”).
From an output high quality standpoint, the water system need to consistently offer water that meets particular USP specifications for chemical and microbiological needs. The chemistry portion of the specification is rather clear-cut and will be met and taken care of as a result of filtration and a variety of ways of ion Trade. The microbiological part, nonetheless, is a challenge. Whilst the chemical composition of water is often established and altered promptly here to be certain a quick reaction to a challenge, the microbiological evaluation is slower and fewer exact. Which means bioburden final results are usually not accessible until finally many days have elapsed, positioning appreciable emphasis upon fantastic design, servicing and monitoring.
4. Any deviation or change from this course of action should be documented and investigated. five. There needs to be a composed method or program for servicing of equipment section must be described within the protocol.
The validation strategy really should be built to ascertain the suitability of the system and provide a radical understanding of the purification system, number of operating circumstances, expected pre-cure, as well as the most likely method of failure.
Even though there isn't any complete microbial requirements for water (in addition to water meant to be sterile), the CGMP laws need that acceptable technical specs be founded and monitored. The specification have to take note of the supposed use with the water; i.
Excellent assurance and high quality Regulate experts, manufacturing supervisors, specialized help staff, validation staff, and all amounts of management who want to achieve a essential knowledge of pharmaceutical water systems
A distribution loop normally has a longer company lifetime than the usual output device. Therefore, the elements are frequently replaced click here at various times.
of minimum movement through the water generation system control of temperature while in the
two. It includes variety of checks built so that you can validate the reliable satisfactory system effectiveness.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now!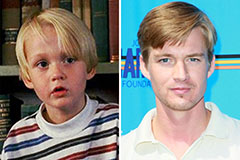 Mason Gamble Then & Now!
Mason Gamble Then & Now! Lisa Whelchel Then & Now!
Lisa Whelchel Then & Now! Richard Dean Anderson Then & Now!
Richard Dean Anderson Then & Now! The Olsen Twins Then & Now!
The Olsen Twins Then & Now!